Computational Modeling of Heart Valves for Surgical Planning
Surgical planning based on computer simulation has the potential to improve the outcome of surgery for heart valve repair. By combining the disciplines of biomechanics, numerical methods, image processing, and computer graphics, we have been developing the capability to produce fast, accurate simulations of heart valves that can be used to address clinically pressing issues in surgical repair of heart valves and, ultimately, can be used for patient-specific surgical planning.
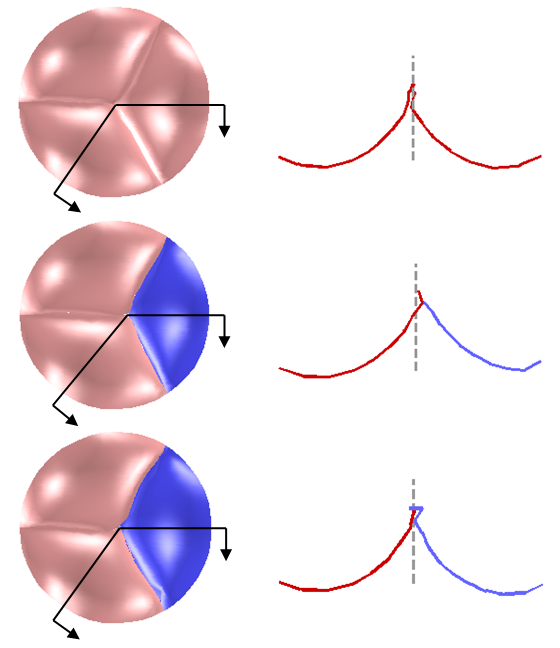
We have developed both a mass-spring model and a finite element model to simulate the nonlinear anisotropic biomechanical behavior of heart valve leaflets (Fig. 1), and we have compared the two methods for speed and accuracy (Table 1) [Hammer et al, Ann Biomed Eng 2011].
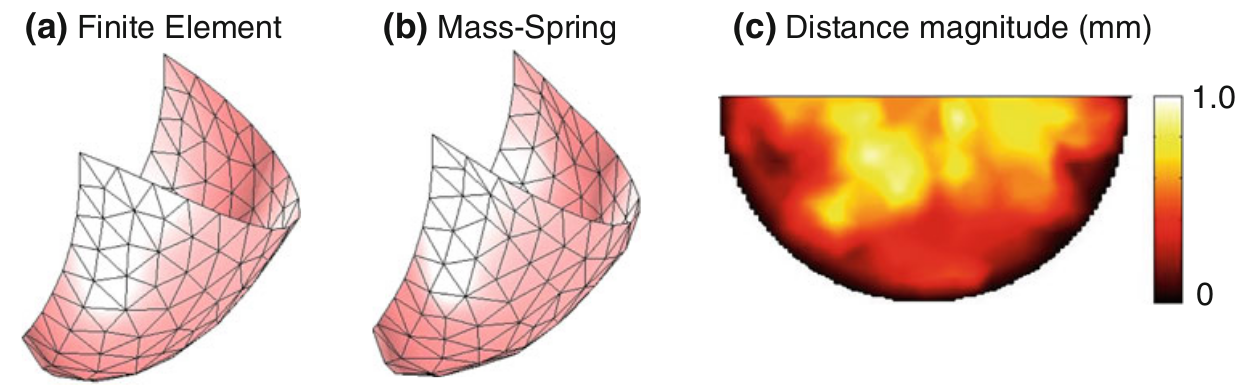
Figure 1. Both finite element and mass-spring models were used to simulate the nonlinear anisotropic behavior of aortic valve leaflets under 80 mmHg of pressure. Agreement between the methods was assessed by plotting the distance between corresponding points on the two deformed meshes.
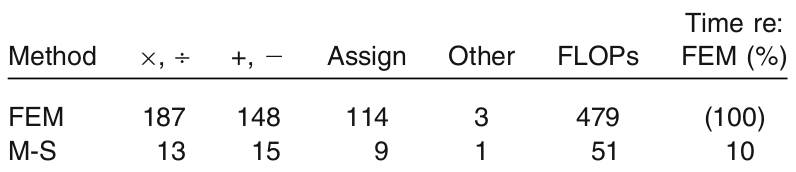
Table 1. Computational cost of the finite element method (FEM) versus the mass-spring model (M-S) for computing internal forces.
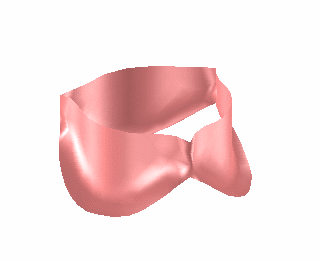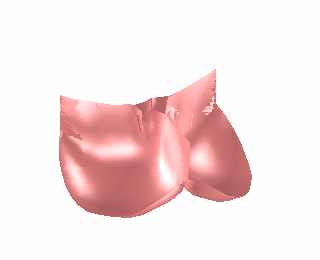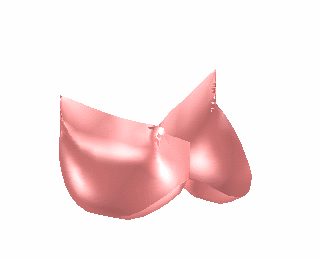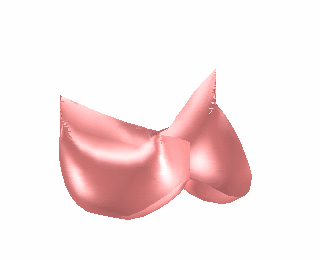
We have used the finite element model of heart valve leaflets to study a common surgical strategy for reconstructing damaged aortic valve leaflets using tissue harvested from the patient’s own pericardium [Hammer et al, J Biomech 2012]. It is difficult for surgeons to determine the size and shape of the leaflet graft in order to achieve a satisfactory repair. We simulated closure of the repaired valve for a range of graft sizes (Fig. 2) and found optimal valve function with a graft that is approximately 25% wider and 25% wider than the native valve leaflets. This oversizing of the graft is necessary to compensate for the decreased distensibility of pericardium relative to native valve tissue.
|
|
Figure 2. Finite element simulation of aortic valve closing (left). Simulations were run for cases where one of the leaflets was replaced with a graft cut from pericardium in a range of different sizes and the ability of the leaflets to seal was assessed (right).
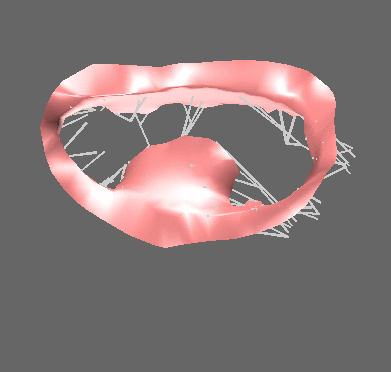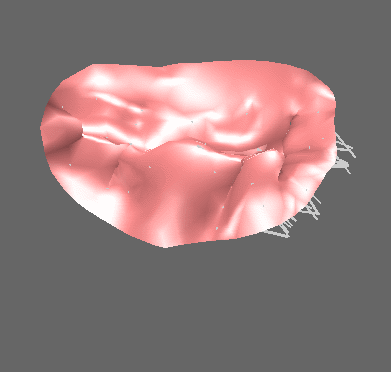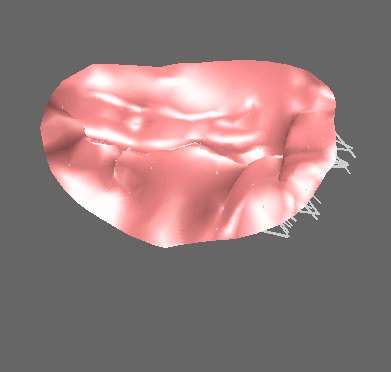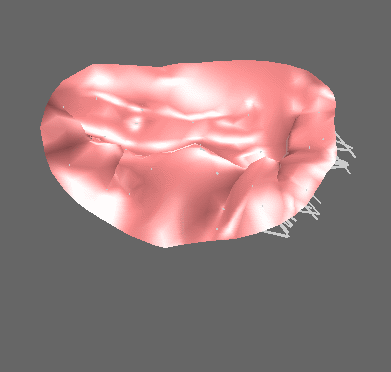
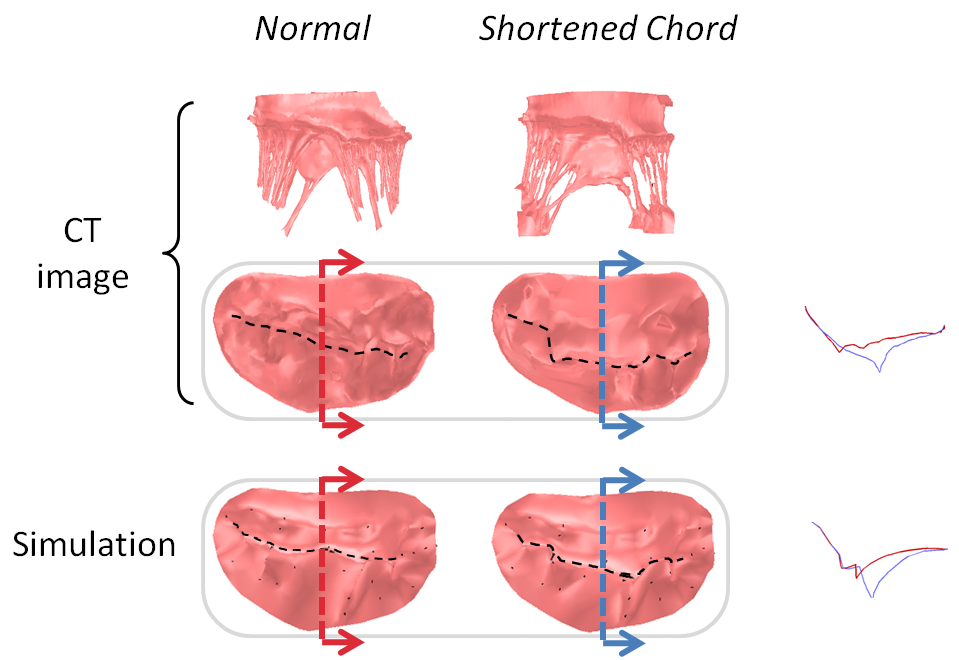
We have applied the mass-spring model of heart valve leaflets to image-based models of the mitral valve (Fig. 3) in order to produce very fast simulations of valve closure – fast enough for pre- or intra-operative surgical planning [Hammer et al, FIMH 2011], [Tenenholtz et al, IROS 2011]. Simulations were used to study the effect of primary and secondary chordae in determining the closed, loaded state of the valve at peak systole and to simulate a simple mitral valve repair technique.
|
|
Figure 3. Mass-spring simulation of closing mitral valve produced from images of porcine heart (left). We simulated a surgical repair consisting of shortening a single primary chord on the posterior leaflet and compared simulation results to images of actual surgery performed on the same heart (right).