Modeling Myocardial Injury During Trans-atrial Intracardiac Procedures
Jeremy W. Cannon*, Pedro J. del Nido**, and Robert D. Howe***
*Department of Mechanical Engineering, MIT
**Department of Cardiovascular Surgery, Children's Hospital Boston
***Biorobotics Laboratory, Harvard University
Support provided by The National Institutes of Health
| MOTIVATION |
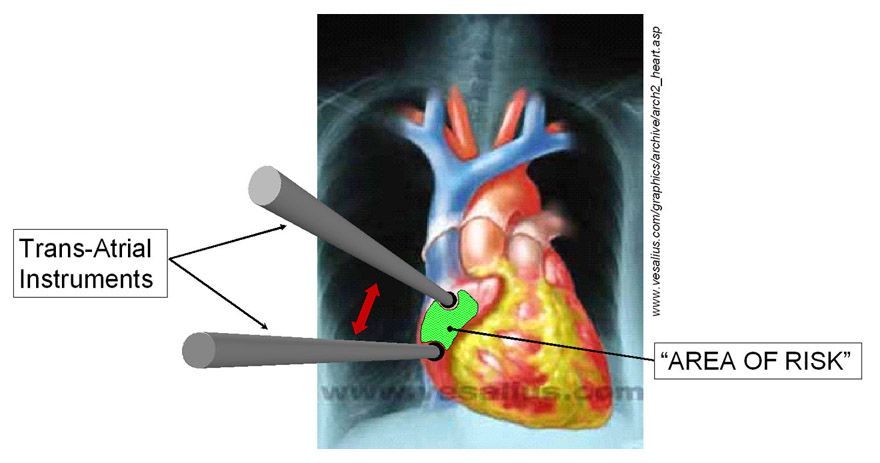 |
| METHOD As an initial approach to limiting this type of intraoperative myocardial injury, a model of the atrial wall was used to study the effects of port/instrument positioning and loading on myocyte injury. The relevant boundary conditions and geometry of the problem are shown in the graphic on the right. This illustration shows the “Region of Risk” between and around trans-atrial ports. This problem was then meshed into a 5300 element quadrilateral planar mesh (FEMAP 8.0) with the myocardial material properties based on the strain energy function by Smaill & Hunter* for both the fiber and cross-fiber directions. The validity of this definition was tested by stretching a single element biaxially and comparing these results to those published by Smaill & Hunter† as shown in the plot below. Then, using the Galerkin Finite Element Method 50 Fiber-Direction and 40 Cross-Fiber Direction port position/displacement combinations (ABAQUS Standard 5.8.1) were solved. 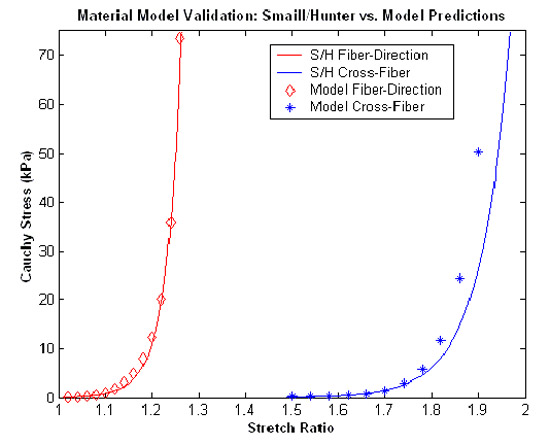 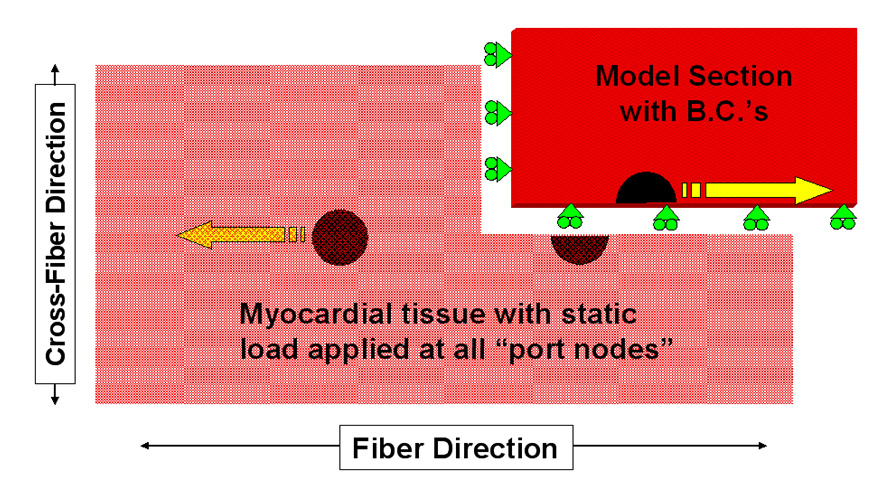 |
| RESULTS These images demonstrate the significant injury sustained by myocytes located between the ports when subjected to high strains. As shown in the graph which summarizes these results, myocyte injury is proportionally less when the ports are oriented in the cross-fiber direction. 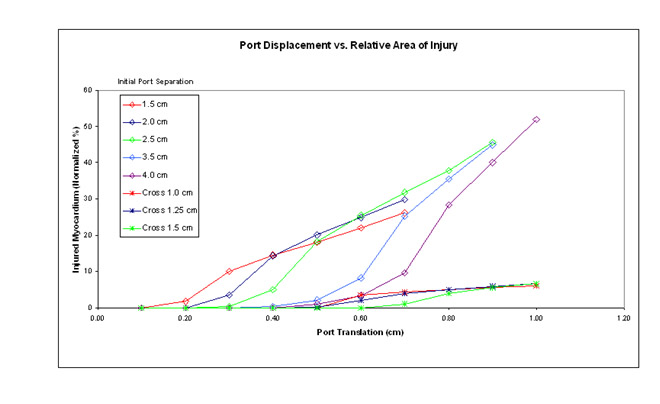 |
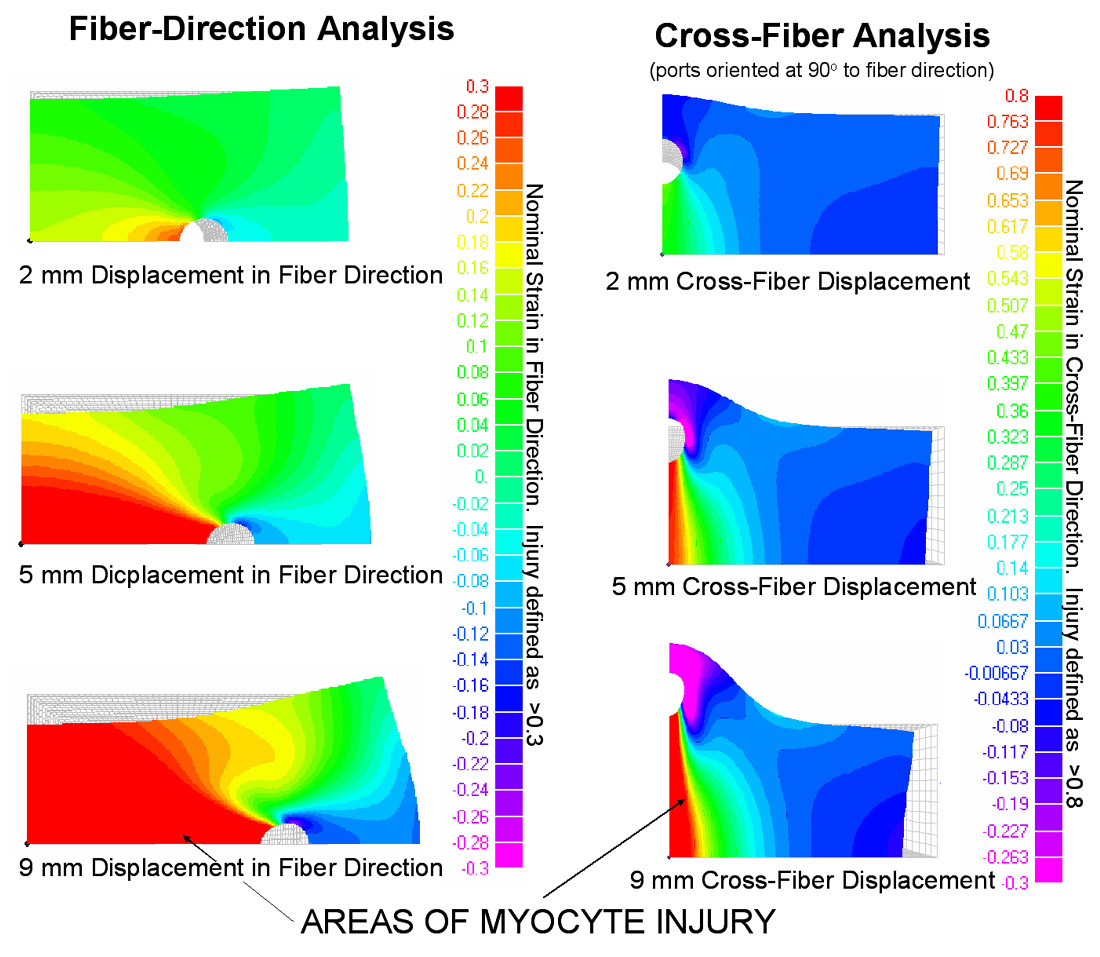 |
| CONCLUSIONS 1) The modeled material fits a myocardial strain energy function over the working range of nominal strain. 2) In the fiber direction, this model predicts reduced myocyte injury with increased initial port separation. 3) This model predicts greater injury over all ranges of forced displacement in the fiber direction compared to the cross-fiber direction. |
| FUTURE WORK Refine material property definition to include fitting atrial-specific strain energy function coefficients based on in vitro tissue property measurements Apply findings to clinical procedures using “smart instruments” fitted with force sensors/feedback actuators which can give warning when approaching soft tissue load limits. |
REFERENCES
J. Cannon, R. Howe, and P. del Nido. (2002). "Modeling Myocardial Injury During Trans-Atrial Intracardiac Procedures" 10th Annual Medicine Meets Virtual Reality Conference. Newport Beach, CA. January, 2002.
†Smaill, B and Hunter, P. (1991). "Structure and function of the diastolic heart." in Theory of Heart: L. Glass, P. Hunter, and A. McCulloch, eds. Springer-Verlag, New York. 1-29.