|
Force Feedback in Surgery: Analysis of Blunt Dissection C.R.
Wagner, N. Stylopoulos*, R. Howe
Support provided by: NSF Fellowship, CIMIT (Center for Integration of Medicine and Innovative Technology) Heart Surgery Grant Paper
presented at: The Tenth Symposium on Haptic
Interfaces for Virtual Environment and Teleoperator Systems, March 24-25,
2002, Orlando
|
| Abstract
|
| 1. Introduction
|
|
2. Methods and Materials |
|
|
2.1. Telemanipulation System |
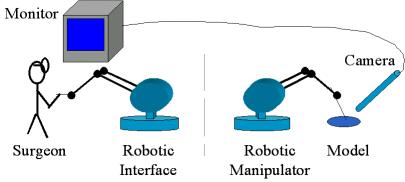
|
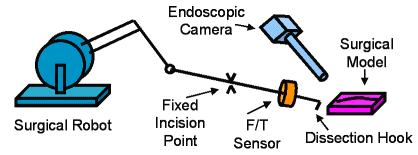
Figure 2: Surgical Setup |
|
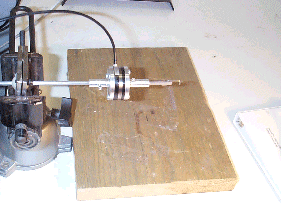
Figure 3: Hook and Force/Torque sensor |
|
|
2.2. Visual Feedback |
| 2.3. Surgical Models
|

|
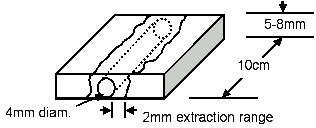
Figure 5: Model Schematic |
|
|
|
|
|
2.4. Experimental Setup |
|
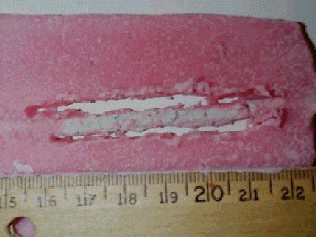
|
|
|
2.5. Measures |
|
2.6 Statistical Analysis |
|
3. Results Force feedback significantly reduced the magnitude of the forces applied
at the instrument tip during dissection. Figure 7 shows a histogram of
the force samples for all subjects and all trials with the visible artery;
samples below 0.1 N are excluded. Subjects applied high force levels for
longer durations when force feedback was not available. Conversely, during
trials with force feedback, less time was spent applying higher forces;
forces above 0.8 N were of negligible duration for 150% force feedback
scaling, and above 1.2 N were negligible for 75% scaling. Further, the
greater the force feedback gain, the less time was spent applying larger
levels of force. These results also apply whether or not the subject can
initially see the artery (Figure 8).
|
|
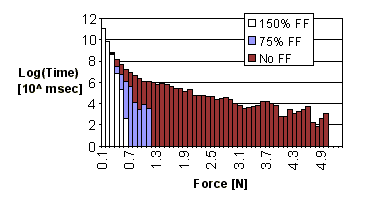
Figure 7: Histogram of forces applied during visible artery trials |
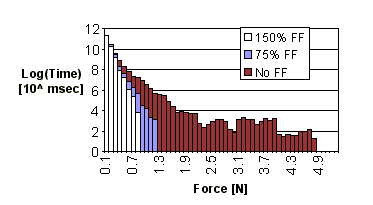
Figure 8: Histogram of forces applied during obscured artery trials |
|
|
|
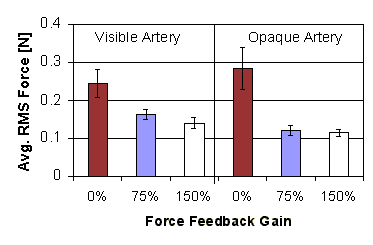
Figure 9: Average RMS force applied versus force feedback gain (error bars show standard error) |
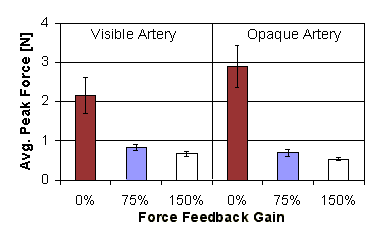
Figure 10: Average peak force applied versus force feedback gain (error bars show standard error) |
|
|
|
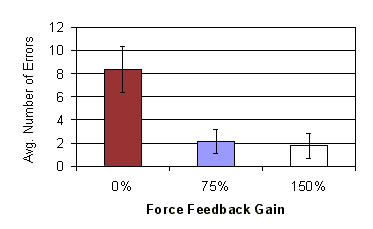
Figure 11: Average number of errors vs. force feedback gain (error bars show standard error) |
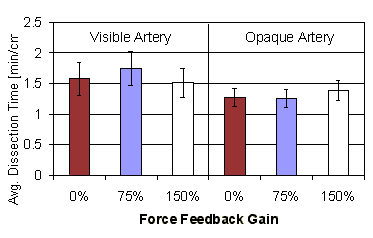
Figure 12: Normalized length dissected vs. force feedback gain (error bars show standard error) |
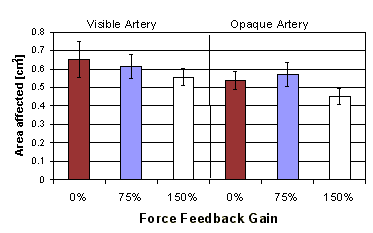
Figure 13: Area affected per cm dissected vs. force feedback gain (error bars show standard error) |
|
|
4. Discussion |
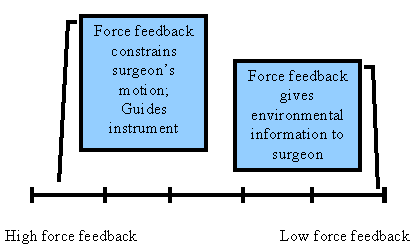
|
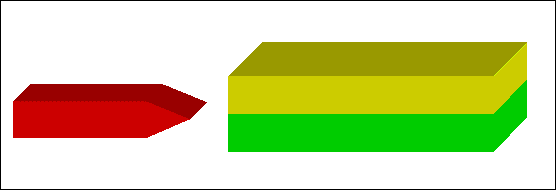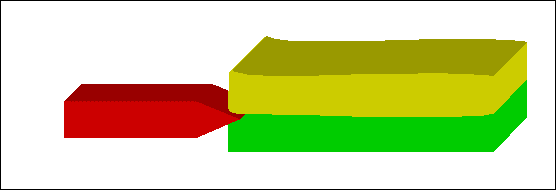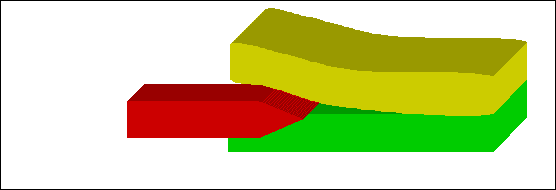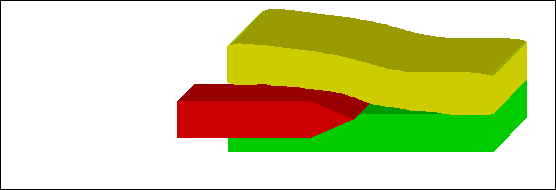
Figure 15: Instrument between two tissues of different stiffness |
|
|
|
|
|
5. References [1] H. Shennib, A. Bastawisy, M. J. Mack, and F. H. Moll, "Computer-assisted
telemanipulation: an enabling technology for endoscopic coronary artery
bypass," Ann Thorac Surg, vol. 66, pp. 1060-3., 1998.
|