Guiding Intracardiac Beating Heart Procedures With Real-Time Three-Dimensional Ultrasound
Paul Novotny MS¹, Jeremy Cannon MD¹, Jeffrey Stoll BS², Robert Howe PhD¹, Pierre Dupont PhD², Ivan Salgo MS MD³, Gerald Marx MD*, Pedro del Nido MD**¹Biorobotics Laboratory, Harvard University
²Robotics, Dynamics and Control Laboratory, Boston University
³Philips Medical Systems
*Cardiology, Children's Hospital, Boston
**Cardiac Surgery, Children's Hospital, Boston
Support Provided by the National Science Foundation and the National Institutes of Health
Motivation
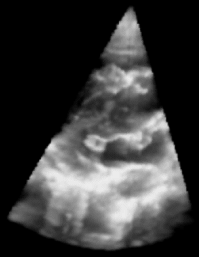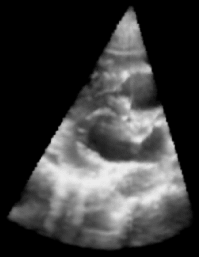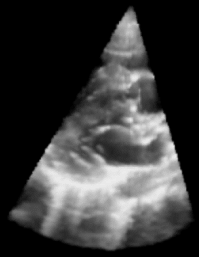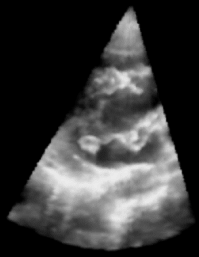 |
| Figure 1: 3D Ultrasound image of a beating heart. |
3D Ultrasound
Traditional 3D ultrasound systems rely on slow offline image processing to construct a 3D volume from a series of 2D ultrasound images. Although, these systems produce good three-dimensional images, they are far from real time, often requiring minutes to construct. The time lag prohibits use in interventional procedures where accurate up to date information is critical for task performance. Phillips Medical Systems has recently released a system that produces 3D ultrasound images in real time (20-25 frames per second). The system consists of a fully sampled two-dimensional array of ultrasound transducers that quickly scans a three dimensional volume. These characteristics suggest that the Philips 3D ultrasound system is a feasible imaging modality for guiding surgical tasks.
Previous Work
In earlier work, our group demonstrated that 3D ultrasound could guide complex surgical tasks in vitro[1]. This was validated by seven surgical trainees performing a range of tasks in a testing tank. The subjects performed these tasks using 2D ultrasound, 3D ultrasound, or endoscopic video for guidance.
 |
| Figure 2: Picture of clip fixation task with corresponding 3D ultrasound image. |
- Bead in Hole
Using an endoscopic grasper, the subject tries to place a 4mm bead into a 5mm hole cut out of a canvas sheet. - Point to Point Navigation
In this task, the surgeons were asked to navigate the tip of a blunt dissection tool from one point in the tank to another.
- Clip Fixation
The goal of this task is to grasp two surgical tubes with an endoscopic tool and then apply a metal clip with an endoscopic clipping tool. This task is a simulation of a common surgical maneuver to attach adjacent tissues.
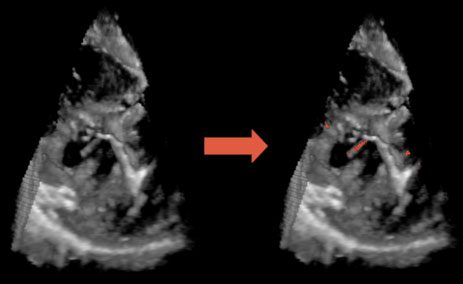
Ultrasound and Surgical Instruments
It is becoming increasingly clear, that instruments
such as endoscopic graspers when immersed in liquids display artifacts and irregularities
that make it difficult to determine the tool’s location, orientation, and geometry.
In addition, the instrument’s shape can appear incomplete due to its orientation or
obstacles in the field. Since the geometry and properties of these instruments are
known a priori, it is feasible to combine this information with the ultrasound image
data to locate and render an enhanced representation of the instrument.
 |
References
[1] Cannon J, Stoll J, Howe R, Salgo I, Knowles H, Dupont P, Marx G, and del Nido P, Real Time 3 Dimensional Ultrasound for Guiding Surgical Tasks, Computer Aided Surgery 2002; In Review.