3D Ultrasound-Guided Robotic Motion Compensation for Beating Heart Intracardiac Surgery
Shelten Yuen MS¹, Sam Kesner MS¹,
Daniel Kettler BS¹, Richard Plowes BS¹, Paul Novotny PhD¹, Robert Howe PhD¹,
Ivan Salgo MS MD²,
Nikolay Vasilyev MD*, Pedro del Nido MD**
|
¹Biorobotics Laboratory, Harvard University ²Philips Medical Systems *Cardiology, Children's Hospital, Boston **Cardiac Surgery, Children's Hospital, Boston Support Provided by the National Institutes of Health |
|
Motivation
|
Traditionally, surgeons have coped with heart motion during cardiac surgery by stopping the heart and using the heart-lung machine to pump and oxygenate the blood. There is great interest in avoiding the heart-lung machine because of its serious side effects. Furthermore, the outcome of a surgery on a stationary heart is challenging to predict. Beating-heart surgery prevents the morbidities associated with the heart-lung machine and potentially improves surgical outcomes by allowing the surgeon to evaluate repair, particularly of valves, during the operation. However, this type of procedure is difficult for surgeons to perform because of heart motion.
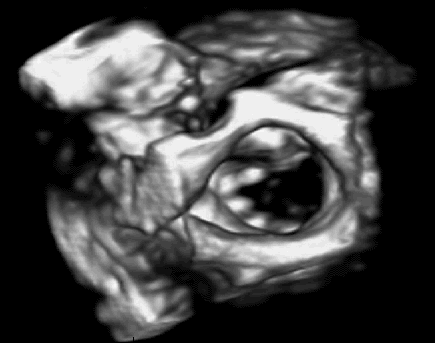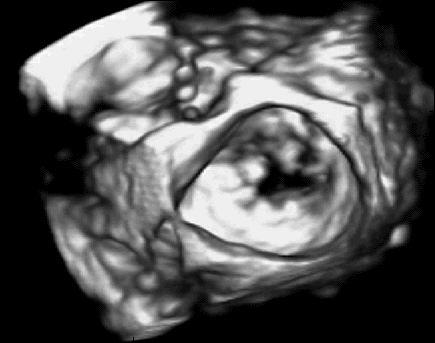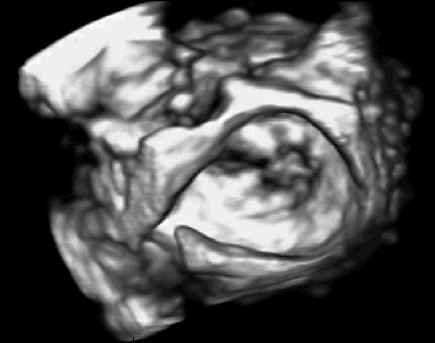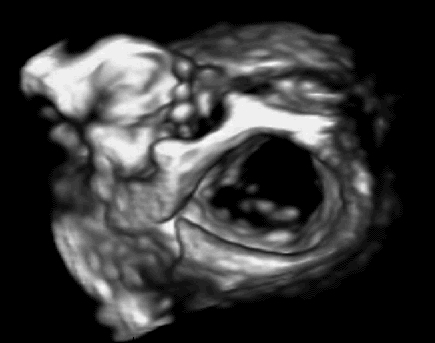
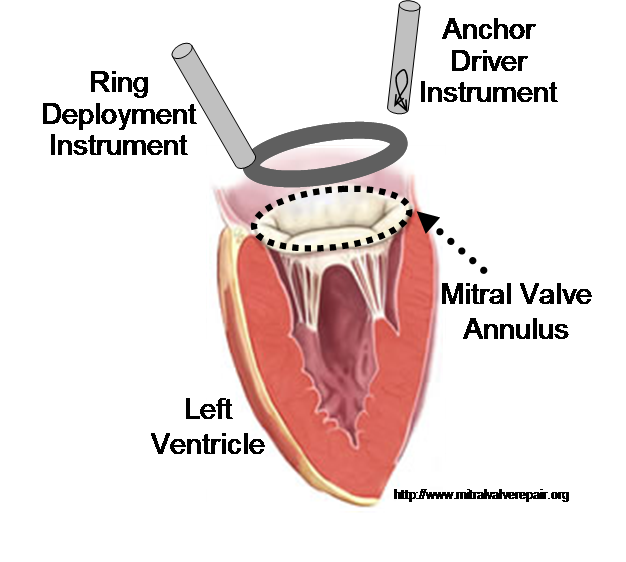
In previous work, we demonstrated that real-time 3D ultrasound is an effective imaging technology for guiding surgeries inside of the heart. In this project, we have developed a 3D ultrasound-guided motion compensation system that tracks heart motion and allows the surgeon to operate on the fast moving structures of the beating heart without risk of damaging them. We focus on the beating heart repair of the mitral valve through mitral annuloplasty. The design of the system is guided by the clinical observation that the rapid motion of the mitral annulus is dominated by translation along a single axis between the left atrium and left ventricle. This allows the use of a simplified 1 DOF motion compensation system that can be used for surgical procedures like the anchor driving found in mitral annuloplasty. |
|
Engineering Challenges
The robotic motion compensation instrument required for this procedure must have sufficient bandwidth to compensate for the fast-moving structures in the heart, as well as a sufficient force output to perform suturing and stapling tasks on heart tissue. It must also be able to operate within the limited confines of the heart. By noting that the motion of the mitral annulus is nearly unixial, we have developed a fast, lightweight, and hand held instrument that meets these surgical requirements (Figure 3). The instrument is capable of delivering surgical anchors to tissue while tracking the fast motion of the mitral annulus [1]. |
|
|
Automatic, real-time segmentation of a tissue target in 3D ultrasound is challenging due to poor shape definition and the number of computations required to process volumes at 28 Hz (~40 MB/sec). We achieve real-time tissue tracking by making use of the instrument to designate a tissue target in the ultrasound volume, essentially by pointing at it. Tracking is turned into a two-stage problem where the instrument is first segmented in the volume through a GPU-based algorithm [2] and then the target is taken to be the tissue directly in front of the instrument [4]. |
|
|
The acquisition and processing of 3D ultrasound volumes introduces a substantial delay in which time the mitral valve can traverse the majority of its trajectory. Left uncompensated, these delays would lead to collisions between the robotic instrument and annulus that would result in tissue damage. To avoid this outcome, we exploit the near periodicity of the mitral valve trajectory to predict its path and hence compensate for time delay [3]. 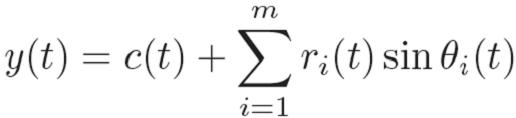 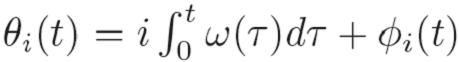 |
|
Motion Compensation Results
Figure 6 shows the resulting motion compensation system. Its use in simulated mitral annuloplasty procedures in a water tank indicate that motion compensation doubles successful anchor depolyment rates while reducing contact forces with the tissue by half [4]. In vivo validation of the system is ongoing.
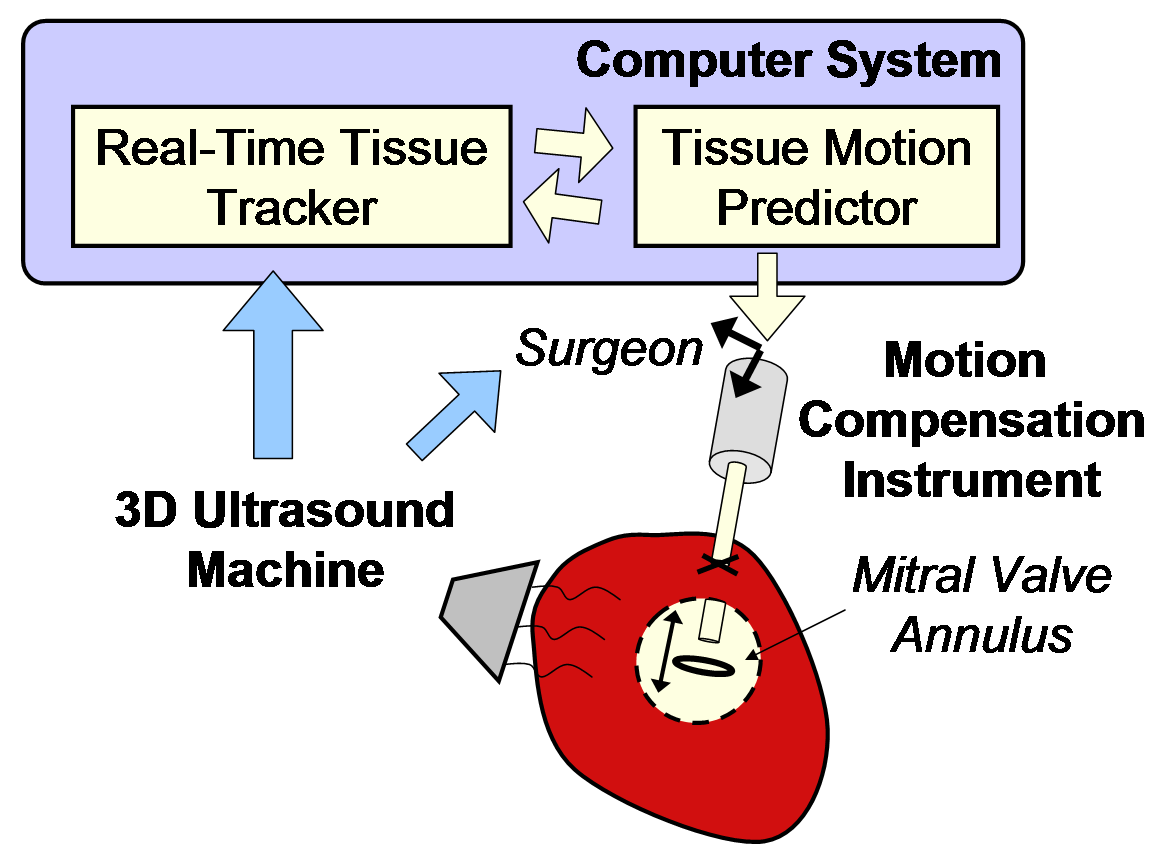 Figure 6: Motion compensation system. |
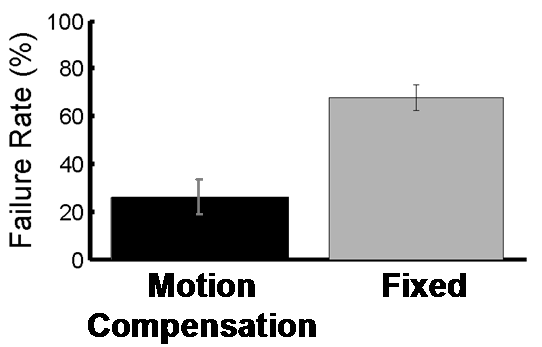 Figure 7: Anchor depolyment failure rates with and without motion compensation. |
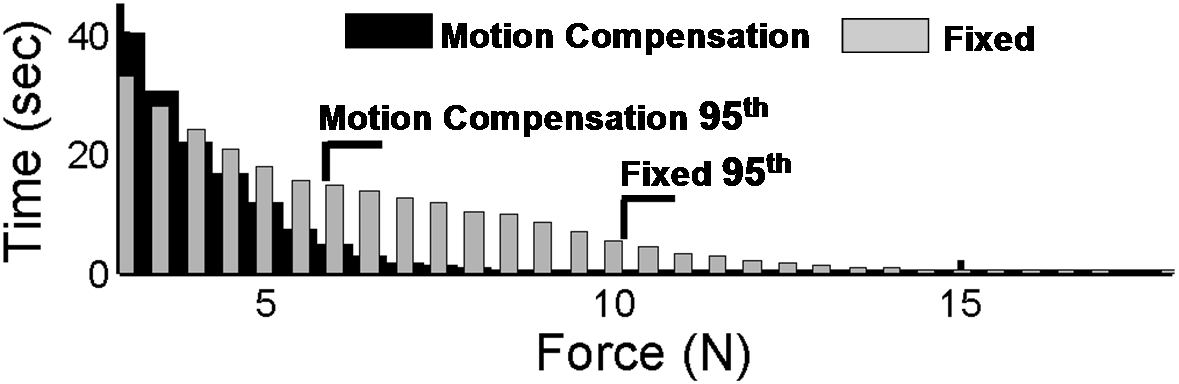 Figure 8: Contact forces during anchor deployment with and without motion compensation. |
References
[1] PM Novotny, JA Stoll, NV Vasilyev, PJ del Nido, PE Dupont, TE Zickler, and RD Howe.
GPU based real-time instrument tracking with three-dimensional ultrasound,
Medical Image Analysis, 11:458-464, 2007.
[2] SG Yuen, DT Kettler, PM Novotny, RD Plowes, RD Howe.
Robotic Motion Compensation for Beating Heart Intracardiac Surgery,
International Journal of Robotics Research, Special Issue on Medical Robotics, 2009. In Press.
[3] SG Yuen, SB Kesner, NV Vasilyev, PJ del Nido, RD Howe.
3D Ultrasound-Guided Motion Compensation System for Beating Heart Mitral Valve Repair,
Medical Image Computing and Computer-Assisted Intervention (MICCAI), New York, NY, Sept. 2008.
[4] SG Yuen, MC Yip, NV Vasilyev, DP Perrin, PJ del Nido, RD Howe.
3D Ultrasound-Guided Motion Compensation System for Beating Heart Mitral Valve Repair,
Medical Image Computing and Computer-Assisted Intervention (MICCAI), London, UK, Sept. 2009. Accepted.
Media Coverage
MIT Technology Review, Operating inside a Beating Heart, R. Kremen, Oct. 21, 2008.
Slashdot, Robotic Surgery On a Beating Heart, Oct. 21, 2008
Gizmodo, New Robot Lets Surgeon Operate on a Heart While it Still Beats, Oct. 22, 2008
MIT Technology Review (Germany), Nahen am schlagenden Herzen, V. Szentpetery, Dec. 2008, page 16.
Please send questions and comments to Shelten Yuen (sgyuen {at} fas harvard edu)
Harvard BioRobotics Laboratory Home